ARTICLE AD BOX
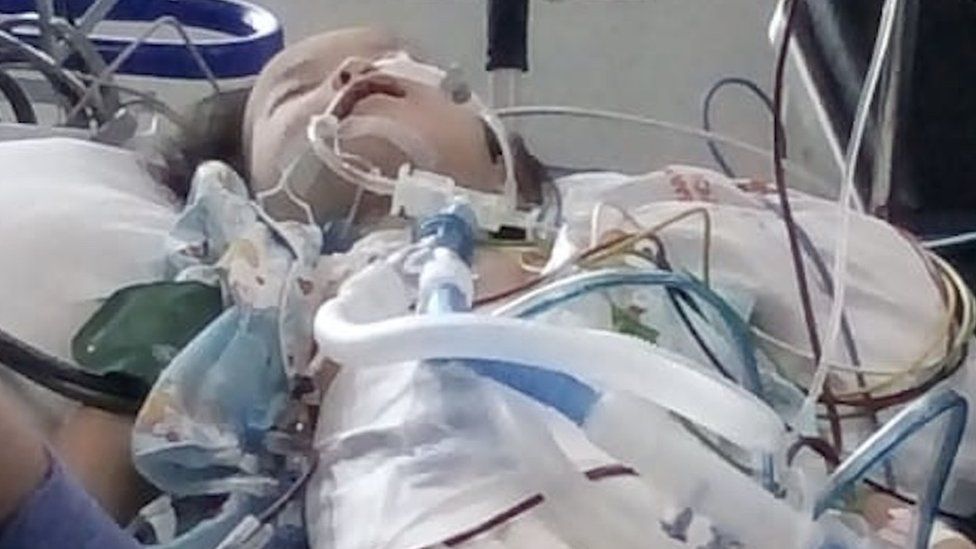 Image source, Handout
Image source, Handout
Four-year-old Nasya was treated in hospital for three weeks but later died
By Valdya Baraputri and Lara Owen
in Jakarta and London
Parents whose children died or were injured by tainted cough syrup have wept with relief after an Indonesian court allowed a class action lawsuit.
"My child's struggle was not in vain," said Nur Asiah, whose four-year-old daughter died last year.
Her family and the relatives of 24 other victims have brought the case against Indonesia's government and eight pharmaceutical companies.
More than 200 Indonesian children have died of acute kidney injury since 2022.
Indonesia is not the only country hit by contaminated cough syrup. About 100 deaths have been reported in The Gambia and Uzbekistan.
An investigation is continuing in Indonesia, but local authorities say so far no evidence shows links with cases in other countries. The World Health Organization (WHO) has issued warnings about six cough syrups made in India and Indonesia.
"I didn't know what I gave to my child was poison," Nur Asiah told the BBC Indonesia ahead of the court's decision.
Her daughter Nasya was prescribed cough syrup after developing a fever last year. She became very sick after consuming the medicine and died three weeks in the hospital later.
The lawsuit seeks compensation of $195,000 for every child killed and about $130,000 for every child injured. Other parents will be allowed to join the lawsuit, their attorney said.
"No amount of compensation will make up for what has happened. It won't bring back my child," Nur Asiah said tearfully.
BBC Indonesia tried to contact the eight companies being sued but not all of them responded before Tuesday.
"It is not appropriate if the responsibility is only placed on the pharmaceutical industry," said the lawyer of PT Afi Farma, whose cough syrup was used by the majority of children in this case, adding that the government should also be held accountable.
Another company PT Universal Pharmaceutical Industries said it had been using the same Indonesian Food and Drug authority (FDA) certified system for about 30 years for their cough syrup brand and it had bought the ingredients from an FDA-approved supplier.
"Honestly, pharmaceutical companies are also victims - victims of a crime by the suppliers of the raw materials," its lawyer said.
A Health Ministry spokesperson said it had been working on a compensation scheme.
"We have tried our best by quickly finding causes, exchanging information with other countries, the WHO, and bringing in antidotes to treat toxic substances."
Indonesian authorities have found that local chemical companies used industrial grade solvent material - Ethylene Glycol and Diethylene Glycol - in the syrup amid a global shortage of pharmaceutical grade solvents. The two substances are typically used in antifreeze solutions for air-conditioners and fridges.

 1 year ago
68
1 year ago
68








 English (US)
English (US)