ARTICLE AD BOX
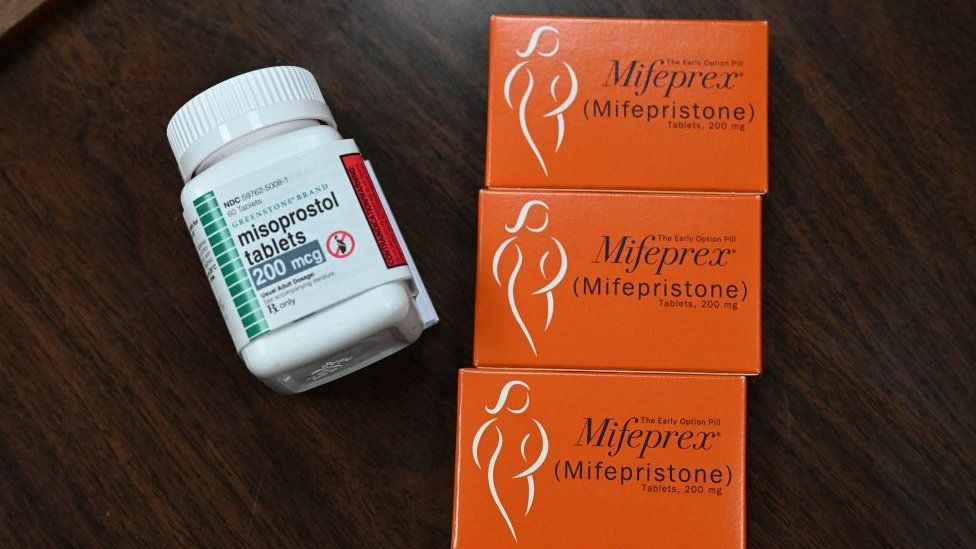 Image source, Getty Images
Image source, Getty Images
The lawsuit claims that the FDA approved mifepristone prematurely.
By Bernd Debusmann Jr
BBC News, Washington
A Texas judge is hearing arguments about whether a commonly used abortion pill should be sold in the US, in a ruling that could curtail access to the drug nationwide.
The pill, mifepristone, can be taken at home and is used in more than half of US pregnancy terminations.
A lawsuit filed by an anti-abortion group in Texas argues that the drug's safety was never properly studied.
The drug has been approved for use for over 20 years.
The case, which will be decided by Judge Matthew Kacsmaryk, an appointee of former President Donald Trump, follows the US Supreme Court's historic overruling last year of the constitutional right to abortion.
The Texas lawsuit, filed by the the Alliance for Hippocratic medicine, an anti-abortion organisation, argued that the US Food and Drug Administration (FDA) approved mifepristone before adequate testing was carried out.
President Joe Biden's administration has responded to the lawsuit, arguing that the drug's approval was well supported by science.
US Department of Justice lawyers wrote that it would be unprecedented for a court to second-guess the FDA's approval and remove a drug from the market, especially after two decades.
Citing security concerns, Judge Kacsmaryk had urged attorneys not to publicise the date of Wednesday's hearing, saying "less advertisement is better".
"This is not a gag order but just a request for courtesy given the death threats and harassing phone calls and voicemails that this division has received," said the judge, according to a teleconference transcript from Friday.
Some media outlets criticised the rare request, citing transparency concerns, and sent a letter to the court. On Monday the judge disclosed the date of the hearing.
Protests were expected outside the federal court in Amarillo.
Julie Marie Blake, senior counsel for the Alliance Defending Freedom, a conservative group that backs the lawsuit, told the BBC's US partner CBS that the organisation is "confident that when any court looks at the law and looks at the science, it will realise that the FDA has completely failed its responsibility to protect women and girls".
Twelve Democratic-led states, including Washington, Arizona, Colorado, Delaware and Connecticut, have filed a separate lawsuit against the FDA seeking to make access to mifepristone easier.
That legal action alleges that the FDA's current regulations concerning the drug are "burdensome, harmful and unnecessary".
If the judge rules the FDA erred in its approval, sales of the drug - one of only two pills used to induce an abortion - could be halted.
But women would still be able to use the other approved abortion drug, misoprostol.
Misoprostol can be used alone and is often the only option in countries where mifepristone is banned for abortions. Some US clinics and providers also only offer misoprostol. The treatment, however, is slightly less effective than a two-drug regimen that also includes mifepristone.
Around 98% of such medication abortions in the US use a combination of mifepristone and misoprostol, according to a study by the Guttmacher Institute, a pro-abortion rights research organisation.
The FDA has reported a total of 26 deaths associated with mifepristone since it was approved in 2000 - a rate of about 0.65 deaths per 100,000 by-pill abortions.
For comparison, the death rate associated with habitual aspirin use is about 15.3 deaths per 100,000 aspirin users.

 2 years ago
38
2 years ago
38








 English (US) ·
English (US) ·